What is a Lead Acid Battery?

Lead Acid Battery are one of the most widely used types of rechargeable batteries, offering reliable power for various applications such as electric vehicles, backup power systems, and industrial equipment. These batteries have been in use for over 150 years, making them a well-established technology in energy storage. In this blog, we will explore the components, types, working principles, and advantages of lead acid batteries.
Despite being heavy and having a relatively low energy density compared to other types of batteries, lead-acid batteries continue to be widely used due to their low cost, robustness, and proven technology.
Components of a Lead Acid Battery
A typical lead acid battery consists of the following key components:
- Electrodes (Plates): These are made up of lead (Pb) and lead dioxide (PbO₂), acting as the positive and negative terminals of the battery.
- Electrolyte: A mixture of sulfuric acid and water is used as the electrolyte. This allows the flow of electric current within the battery.
- Separator: The separator prevents the positive and negative plates from touching each other while allowing ions to pass through.
- Casing: The battery is housed in a durable plastic or rubber case to prevent leaks and provide protection.

Applications of Lead Acid Batteries
Lead acid battery are utilized in a assortment of businesses due to their cost-effectiveness and reliability:
Automotive Industry: These batteries are commonly utilized in cars, trucks, and cruisers to control the start, lights, and electrical systems.
Electric Vehicles: Lead corrosive batteries are utilized in electric scooty, e-rickshaws, e-bke and bicycles, advertising an reasonable control source.
Renewable Vitality Capacity: They are utilized for putting away vitality in sun based control frameworks and wind vitality applications.
Uninterruptible Control Supply (UPS): Lead corrosive batteries give reinforcement control for servers, broadcast communications, and therapeutic gear.
Types of Lead Acid Battery
Flooded Lead Acid Battery
Flooded lead acid batteries are the most traditional and widely used type. These batteries are filled with a liquid electrolyte made of sulfuric acid and require regular maintenance.
Key Characteristics:
- Maintenance: They require periodic refilling with distilled water to maintain electrolyte levels.
- Venting: These batteries emit gases (hydrogen and oxygen) during charging, which is why they need proper ventilation.
- Applications: Commonly used in automotive vehicles, backup power supplies, and renewable energy systems.
- Cost: Typically more affordable compared to sealed lead acid batteries.
Subtypes of Flooded Lead Acid Batteries:
- Starter Batteries: Used primarily in cars, trucks, and motorcycles for ignition and short-term high-current supply.
- Deep Cycle Batteries: Designed to provide a steady amount of current over an extended period, typically used in solar energy storage, electric vehicles, and marine applications.
Sealed Lead Acid Battery (VRLA)
Sealed lead acid batteries, also known as Valve Regulated Lead Acid (VRLA) batteries, are maintenance-free as the electrolyte is immobilized. They are sealed and do not require refilling, making them safer and more convenient to use.
Key Characteristics:
- Maintenance-Free: No need for electrolyte refilling, making them hassle-free.
- Low Gassing: Since they are sealed, these batteries release minimal gas, so ventilation requirements are lower.
- Applications: Commonly used in uninterruptible power supplies (UPS), medical devices, security systems, and backup power.
- Cost: Generally more expensive than flooded lead acid batteries due to the convenience of being maintenance-free.
Subtypes of Sealed Lead Acid Batteries:
- Absorbent Glass Mat (AGM) Batteries:
- In AGM batteries, the electrolyte is absorbed into a glass mat, preventing leakage and improving charge efficiency.
- Key Features: Higher energy density, faster recharging, and increased durability compared to flooded lead acid batteries.
- Applications: Used in motorized vehicles (including motorcycles and RVs), solar energy systems, and UPS systems.
How Does a Lead Acid Battery Work?
---
Lead acid batteries store electrical energy through a reversible chemical reaction between the lead plates and the sulfuric acid. When the battery discharges, lead dioxide on the positive plate and lead on the negative plate react with the sulfuric acid to form lead sulfate (PbSO₄) and water. When recharging, this reaction is reversed, and the lead sulfate converts back into lead and lead dioxide.
Working Principle:
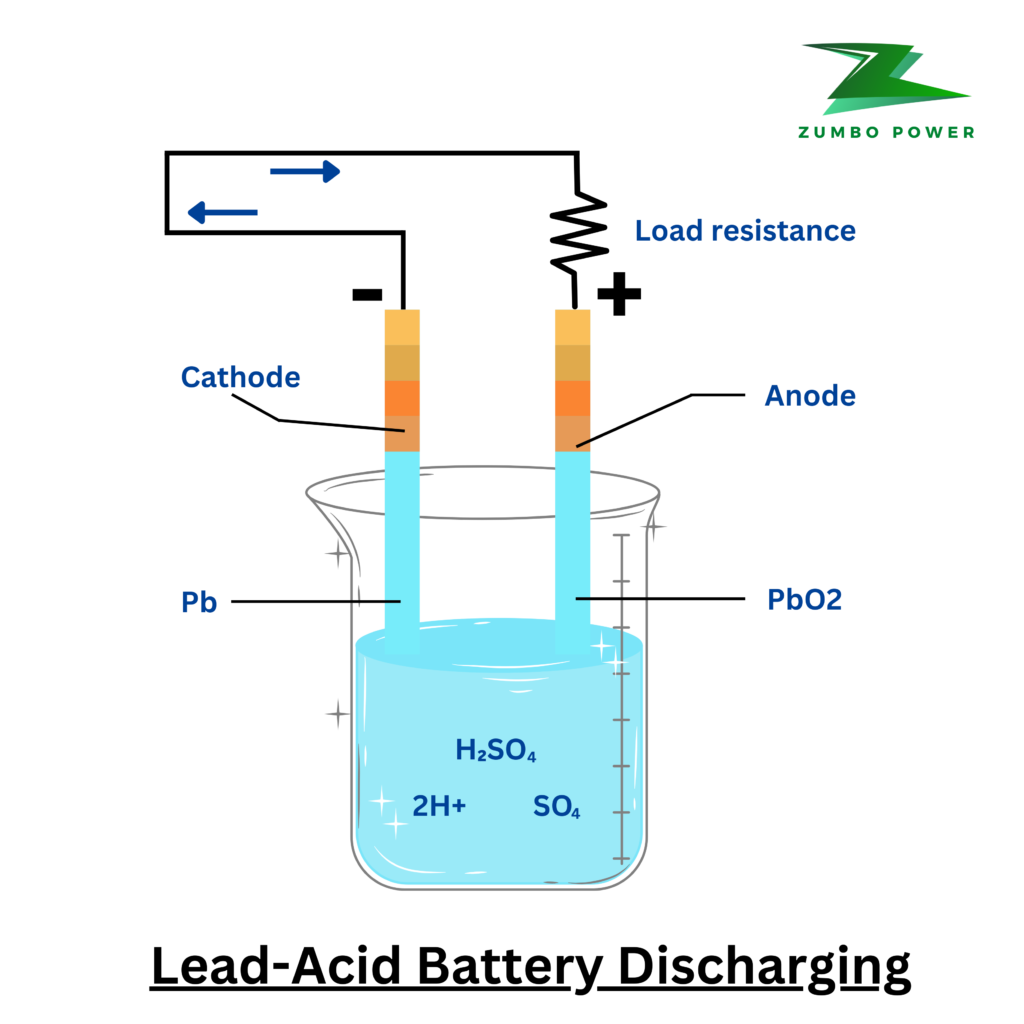
Discharging Process: When the battery is in use (discharging), the following reaction takes place:
- At the positive plate: Lead dioxide reacts with sulfuric acid to form lead sulfate (PbSO₄), releasing electrons.
- At the negative plate: Lead also reacts with sulfuric acid, forming lead sulfate and releasing electrons.
- These reactions create an electron flow, generating electrical current for devices like cars, scooters, or backup systems.
Overall Chemical Reaction During Discharge: PbO2+Pb+2H2SO4→2PbSO4+2H2O (Lead dioxide + Lead + Sulfuric Acid → Lead Sulfate + Water)
Charging Process: During recharging, the external power source reverses the chemical reaction:
- Electrical energy breaks down lead sulfate on both plates, returning it to lead dioxide on the positive plate and pure lead on the negative plate.
- Sulfuric acid concentration increases as the electrolyte regenerates, allowing the battery to store energy again.
Overall Chemical Reaction During Charge: 2PbSO4+2H2O→PbO2+Pb+2H2SO4 (Lead Sulfate + Water → Lead Dioxide + Lead + Sulfuric Acid)
